Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank


Avioq Bio-tech Co., ltd.
We are professional manufacturer that focuses on the R&D, manufacturing and marketing of in-vitro diagnose reagents of biomedical field.
Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank

|
Intended Use
This kit is used to qualitatively detect the novel coronavirus (SARS-CoV-2) nucleocapsid protein antigen in human nasopharyngeal swab samples in vitro. In the acute phase of infection, antigens are usually detectable in upper respiratory tract specimens.
Test Procedure |
| 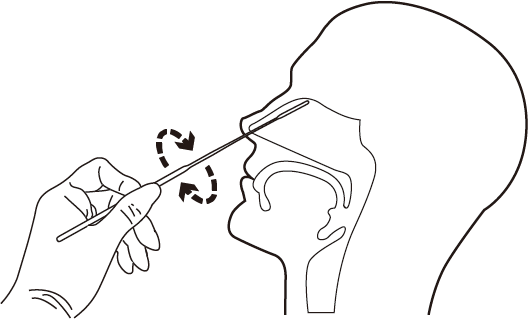 |
| 1.Peel off the sealed aluminum paper of sample extraction tube. | 2.Open the package of nasopharymgeal swab. | 3.Insert the nosal swabs into nose, rotate and push swabs softly. |
| 4.Insert the swab into the extraction tube and roll the swab at least 6 times. Then press the head against the bottom and side of the extraction tube. | ||
| 5.Leave the swab in the extraction tube for 1 minute. Then remove the swabs from tube and cover the dropper tips. | 6.Open the pouch of detection card and place it on a level surface. | 7.Reverse the sample extraction tube, and add 4 drops of extracting solution into loading area. The result should be read in 15 minutes. |
Package
| Speification | 25 tests/kit |
| Test quantity per carton | 1500 tests |
| Box quantity per carton | 60 boxes |
| Carton size&Measurement | 87*38*43cm 0.142cbm |
| G.W per carton | 21.24kgs |
| If you need more package information,please contact with us! | |
1.Delivery date
A:leading time of sample order is about 3-5 wordays.
2.Shipment
A:We usually ship by DHL, UPS, FedEx. It usually takes 3-5 days to arrive.Airline and sea shipping also optional.
3.If you accept OEM?
A:yes
4.Will you help me register the products in my country?
A:we will offer support to help you to register the products until the registration is finished.
5.Do you have MOQ?
Low MOQ, 1pc for sample checking is available
More information please visit website:www.avioqbio.com